The individual who appears is for illustrative purposes. The person depicted is a model and not a healthcare professional.
Reimbursement
Reimbursement resources for every practice
Bracco understands the importance of reimbursement in the sustainability and financial viability of any cardiac PET program. That’s why our service doesn’t stop at clinical training. Our expert team has helped providers like you navigate today’s challenging and ever-changing reimbursement landscape. Through customized business case tools, educational webinars, and peer support, Bracco has everything you need to succeed!
Subscribe to receive exclusive
resources and important
updates from Bracco.
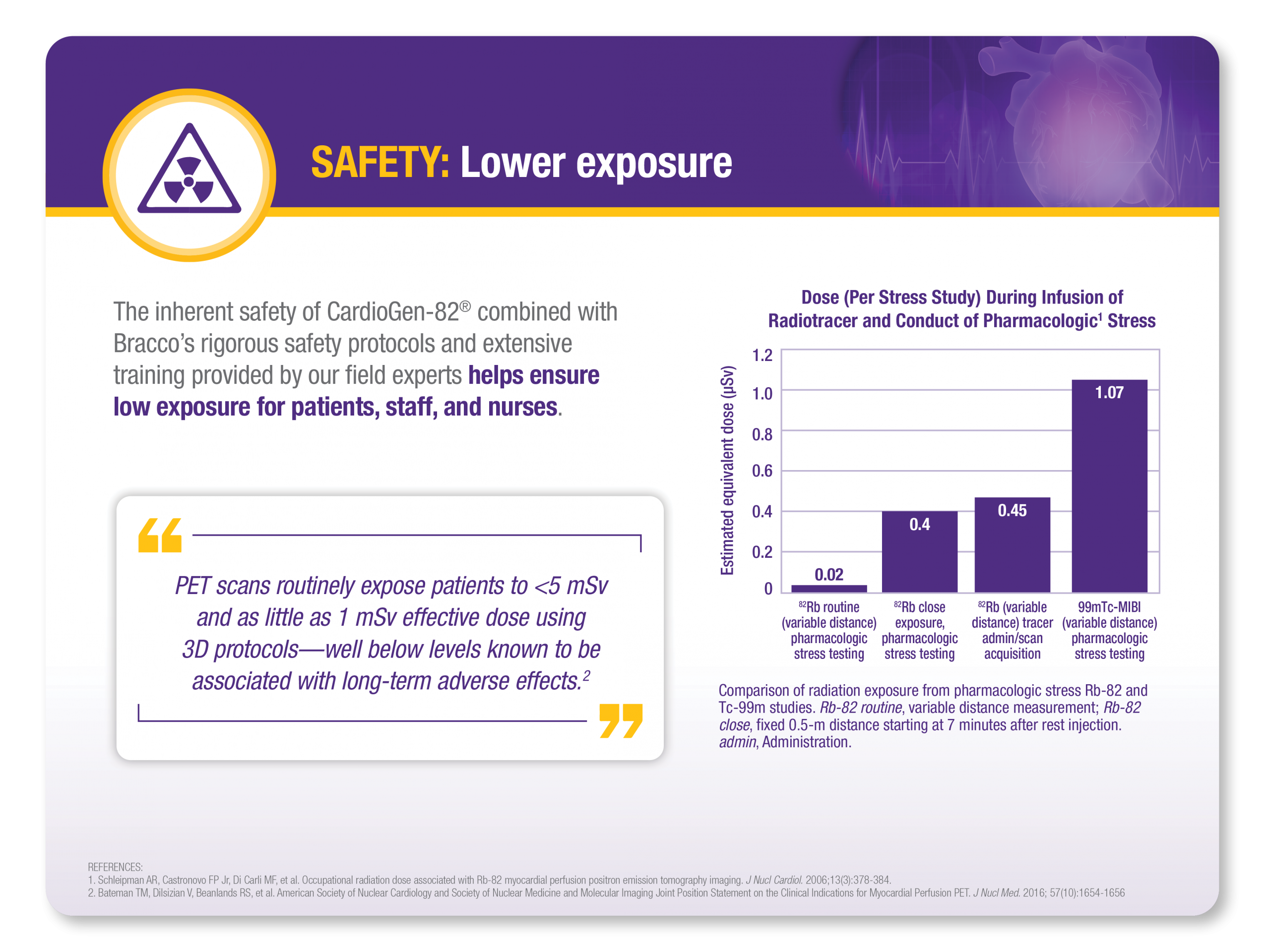
CARDIOGEN-82® (rubidium Rb 82 generator)
INDICATION
CARDIOGEN-82 is a closed system used to produce rubidium Rb 82 chloride injection for intravenous administration and is indicated for Positron Emission Tomography (PET) imaging of the myocardium under rest or pharmacologic stress conditions to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease.
IMPORTANT SAFETY INFORMATION
WARNING: HIGH LEVEL RADIATION EXPOSURE WITH USE OF INCORRECT ELUENT AND FAILURE TO FOLLOW THE ELUATE TESTING PROTOCOL
High Level Radiation Exposure with Use of Incorrect Eluent
Patients are exposed to high radiation levels when the CardioGen-82 generator is eluted with the incorrect eluent due to high Sr 82 and Sr 85 breakthrough levels.
- Use only additive-free 0.9% Sodium Chloride Injection USP to elute the generator
- If an incorrect solution is used to elute CardioGen-82:
- Immediately stop the patient infusion;
- Evaluate the patient’s radiation absorbed dose and monitor for the effects of radiation to critical organs such as bone marrow;
- Permanently discontinue use of the affected generator.
Excess Radiation Exposure with Failure to Follow the Eluate Testing Protocol
Excess radiation exposure occurs when the levels of Sr 82 or Sr 85 in the rubidium Rb 82 chloride injection exceed specified limits.
- Record each generator eluate volume, including waste and test volumes, and keep a record of the cumulative eluate volume
- Strictly adhere to the generator eluate testing protocol, to minimize the risk of excess radiation exposure, including daily testing and additional testing at Alert Limits
- Stop using the generator if it reaches any of its Expiration Limits: 17 L for the generator’s cumulative eluate volume, or 42 days post generator calibration date, or an eluate Sr 82 level of 0.01 μCi /mCi Rb 82, or an eluate Sr 85 level of 0.1 μCi /mCi Rb 82
WARNINGS AND PRECAUTIONS
High Level Radiation Exposure with Use of Incorrect Eluent
Use only additive free 0.9% Sodium Chloride Injection USP to elute the generator. Apply the provided saline tag to the additive free 0.9% Sodium Chloride Injection USP container before use. Additives present in other solutions (particularly calcium ions) expose patients to high levels of radiation by causing the release of large amounts of Sr 82 and Sr 85 into the eluate regardless of the generator’s age or prior use. Immediately stop the patient infusion and discontinue use of the affected CardioGen-82 generator if the incorrect eluent is used and evaluate the patient’s radiation absorbed dose and monitor for the effects of radiation to critical organs such as bone marrow.
Excess Radiation Exposure with Failure to Follow Testing Protocol
Excess radiation exposure occurs when the Sr 82 and Sr 85 levels in rubidium Rb 82 chloride injections exceed the specified generator eluate limits. Strictly adhere to the eluate testing protocol to minimize radiation exposure to the patient. Stop using the rubidium generator when the expiration limits are reached.
Risk Associated with Pharmacologic Stress
Pharmacologic induction of cardiovascular stress may be associated with serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, and cerebrovascular events. Perform pharmacologic stress testing in accordance with the pharmacologic stress agent’s prescribing information and only in the setting where cardiac resuscitation equipment and trained staff are readily available.
Cumulative Radiation Exposure: Long-Term Risk of Cancer
Rubidium Rb 82 chloride injection, similar to other radiopharmaceuticals, contributes to a patient’s overall long-term cumulative radiation exposure which is associated with an increased risk of cancer. Use the lowest dose of rubidium Rb 82 chloride injection necessary for imaging and ensure safe handling to protect the patient and health care worker. Encourage patients to void as soon as a study is completed and as often as possible thereafter for at least one hour.
ADVERSE REACTIONS
Radiation Exposure
High level radiation exposure to the bone marrow has occurred in some patients due to Sr 82 and Sr 85 breakthrough in the eluate when an incorrect solution was used to elute the rubidium Rb 82 generator. Excess radiation exposure has occurred in some patients who received rubidium Rb 82 chloride injections at clinical sites where generator eluate testing appeared insufficient.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for CardioGen-82 (rubidium Rb 82 generator) including boxed WARNING.
CARDIOGEN-82 is manufactured for Bracco Diagnostics Inc., Princeton, NJ 08540, by GE Healthcare, Medi-Physics, Inc., South Plainfield, NJ 07080.
CARDIOGEN-82 is a registered trademark of Bracco Diagnostics Inc.
When HEARTSEE is mentioned, add the following statement:
HEARTSEE is a trademark of The University of Texas Health Science Center at Houston on behalf of the Board of Regents of The University of Texas System and used under a license by Bracco Diagnostics Inc.
When WE ARE CARDIAC PET® is mentioned, add the following statement:
WE ARE CARDIAC PET is a registered trademark of Bracco Diagnostics Inc.